INTRODUCTION
Aflatoxins are the most intensively researched group of mycotoxins, and the effects of aflatoxins on broiler productivity have been previously reviewed (Kensler et al., 2011; Yunus et al., 2011; Monson et al., 2015).
Previous studies have shown that aflatoxins have a variety of negative effects, including slower growth (Magnoli et al., 2011), carcinogenic effects, immunosuppression, and increased susceptibility to disease (Rawal et al., 2010). Among the known aflatoxins, aflatoxin B1 (AFB1) is the most potent hepatotoxin (Wogan et al., 1992; Rawal et al., 2010) and is classified as a Group I carcinogen by the International Agency for Research on Cancer (2012).
Resistance to aflatoxin in chickens is higher than in other animals and susceptibility varies with breed, species, age, dose and length of exposure (Monson et al., 2015).
Despite numerous prior studies, the dietary concentrations and length of exposure to AFB1 have greatly varied between studies, making it difficult to define a time-and dose-dependent effect on such parameters as weight gain, effects on the liver, and changes in blood chemistry.
This study designed to establish a relationship between the dosage and length of exposure to purified AFB1 on the growth, biochemical parameters, and liver histopathology.
In broiler chicks, the results of which will provide parameter for the toxic effect provoked by AFB1.
MATERIALS AND METHODS
Mixed-gender broiler chickens (Ross 308) at 1d of age were obtained from a commercial hatchery (Join Co., Ltd, Korea). They were housed in an isolator (Threeshine Inc., Daejeon, Korea) which was equipped with an electrically heated, negative pressure, forced ventilation unit as well as a feeder and water trough for each cage. The brooding temperature was set at 33∼35℃ for day 0 then decreased gradually to 23∼21℃ until day 21 and was maintained there until the end of the experiment. The temperature and relative humidity were monitored every 3 hours. Every effort was made to achieve and maintain the optimum temperature and relative humidity according to the Ross Broiler Management Handbook (2014). The light regime began with 24 hours a day for 2 days, then decreased by 30 minutes every other day until it reached 20 hours of light per day, which was maintained until the end of the experiment.
The AFB1-contaminated diets were prepared according to the method described by Kaoud (2013). Briefly, crystalline AFB1 (Cayman chemical, MI, USA) was dissolved in methanol (1 mg AFB1/mL in methanol) and subsequently added to a commercial crumble diet (AT-bioco., Ltd, Ochang, Korea), which was formulated to meet the nutrient requirements of broilers from 1 to 21 days of age (crude protein: above 22.0 %, metabolizable energy (ME): 3,100 kcal/kg). The methanol was then evaporated at room temperature, and the AFB1-treated diet was refrigerated until needed. The control group was fed the same commercial diet without the AFB1. The four diets contained the following: Control (0 mg AFB1), 0.5 mg, 1.0 mg and 2.0 mg AFB1 per kg of feed. All chicks were provided ad libitum access to the diet and water throughout the study.
All experimental procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Bansuk LTC (permission no.: 2016-007).
Broiler chicks were randomly selected from each treatment group at 7 (n=10), 14 (n=10) and 21 days (n=20) post-feeding (DPF) and weighed. Blood samples were collected by heart puncture or from the wing veins. After necropsy, the livers and spleens were removed and weighed immediately. The relative weights of the livers and spleens were calculated per gram of body weight.
The blood samples were allowed to coagulate at room temperature, centrifuged, and the sera collected. All parameters were evaluated using an automatic analyzer (Hitachi 7020 automatic analyzer, Tokyo). The examined parameters included glucose, albumin, total protein (TP), globulin, cholesterol, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine, calcium, and phosphorus.
For the histopathological analysis (n=5 per group), the left lobe of the liver was immediately fixed in a 10% neutral buffered formalin solution followed by routine processing for paraffin wax embedding. The liver tissues were cut into 5-μm-thick sections. After being deparaffinized, sections were stained with Harris modified hematoxylin and eosin solution (Sigma-Aldrich).
Paraffin-embedded liver tissues were cut into 5-µm thick sections. Deparaffinized sections were stained for 60 min with Picro-Sirius red solution (Sigma-Aldrich) and then rinsed three times with 0.5% acetic acid. Sections were dehydrated with absolute alcohol. Fibrosis fibers were quantified in Sirius Red-stained sections of the liver using a ProgRes C5 digital camera (Olympus DP72) attached to a light microscope (Olympus BX53/U-LH 100HG, Olympus Corp., Tokyo, Japan) using at least three birds per group (three areas/section), and semi-quantified using Image J software (NIH, Bethesda, MD, USA). We measured the light polarized Sirius Red area and divided by the total area [(light polarized area/total area) × 100], and the results are shown as means±standard error of the mean (SEM).
RESULTS
The initial average body weight per chick was 39.6±0.2 g. The body weights of the broiler chicks showed no significant differences among the treatment groups on day 7 of AFB1 feeding (Table 1). On 14 and 21 DPF in the 2.0 mg AFB1 group, the body weights were significantly lower than that of the other treatment groups (p<0.01, p<0.001, respectively) (Table 1). Although no significant difference occurred among the control, 0.5 and 1.0 mg AFB1/kg groups, the body weight of the AFB1-fed broilers was generally lower than their counter parts fed the control diet (Table 1).
The relative liver weights began to be affected by AFB1 at 14 days DPF, showing a significant increase in the group fed 2.0 mg/kg of feed (Table 2). On 21 DPF, the relative liver weights were significantly increased in a dose-dependent manner (Table 2).
The relative spleen weights increased significantly in response to 2.0 mg AFB1/kg of feed at 21 days DPF (p<0.001) when compared to those fed the control diet, 0.5 and 1.0 mg AFB1/kg of feed (Table 2).
Fig. 1 shows the effects of dietary AFB1 on serum biochemical parameters.
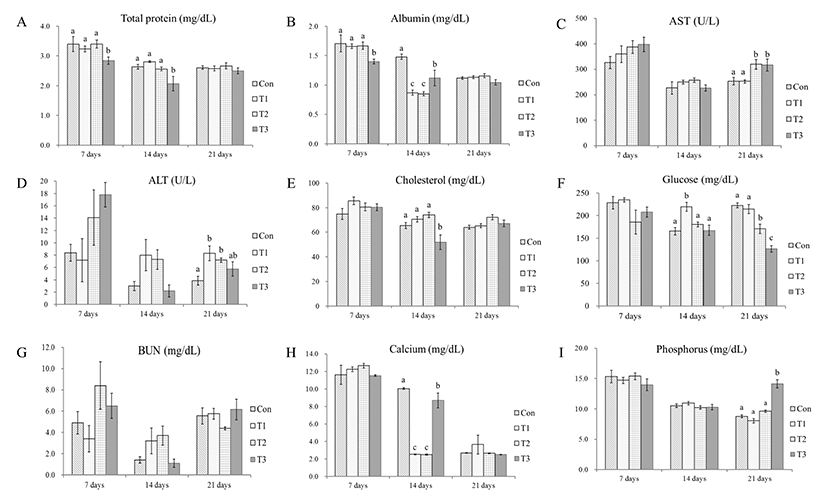
Serum total protein (TP) levels were significantly decreased on 7 and 14 DPF in the group treated with 2.0 mg AFB1/kg of feed compared with the other treatment groups (p<0.05), but the change was not significant at 21 DPF (Fig. 1A).
Albumin levels were significantly decreased in the group treated with 2.0 mg AFB1/kg of feed compared with the other treatment groups at 7 DPF (p<0.05) and compared to the control group at 14 DPF (p<0.01) (Fig. 1B).
AST and ALT levels were not significantly changed at 7 and 14 DPF, but significant changes appeared at 21 DPF. AST levels were significantly increased in the 1.0 and 2.0 mg AFB1/kg of feed groups compared with the control group at 21 DPF (p<0.05) (Fig. 1C). ALT levels in all of the AFB1-contained feed groups were higher than the control group and significantly increased in the groups treated with 0.5 mg (p< 0.01) and 1.0 mg AFB1/kg of feed (p<0.05) (Fig. 1D).
Serum cholesterol levels were significantly decreased in the chicks treated with 2.0 mg AFB1/kg of feed compared with other groups at 14 DPF (p<0.05) (Fig. 1E).
Serum glucose levels showed changes at 14 DPF in the groups treated with 0.5 mg AFB1/kg of feed compared with the other treatment groups (p<0.01, p<0.001). At 21 DPF, glucose levels in the AFB1-treated groups decreased in a dose-dependent manner (p<0.001) compared with the control and 0.5 mg AFB1/kg groups (Fig. 1F).
BUN levels did not show any significant changes (Fig. 1G).
Serum calcium levels were significantly decreased in the groups treated with 0.5, 1.0 (p<0.001) and 2.0 mg AFB1/kg of feed (p<0.05) compared to the control group at 14 DPF (Fig. 1H).
Serum phosphorus levels were significantly increased in the group treated with 2.0 mg AFB1/kg of feed compared with the other groups at 21 DPF (p<0.001) (Fig. 1I).
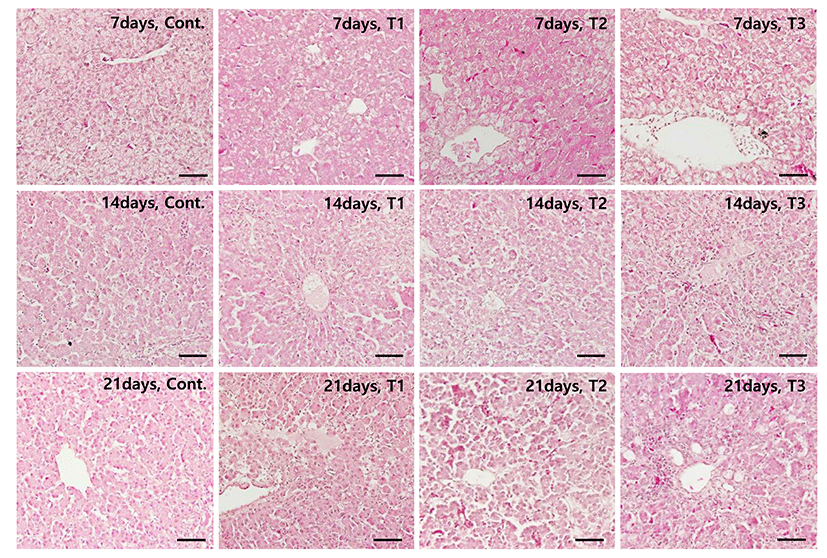
There were no visible liver lesions in the control group birds. Livers from the birds consuming AFB1-containing diets, however, showed significant lesions, such as hemorrhage, hepatocyte necrosis, inflammatory cell infiltration, and fatty degeneration. Hepatocyte necrosis and inflammatory cell infiltration both appeared to increase in severity in a dose- and time-dependent manner (Fig. 2). Fig. 2 shows the histopathological changes from aflatoxin B1-contaminated feed at each time point. To evaluate fibrosis, Sirius red staining was performed on the liver sections at 21 DPF (Fig. 3). The control group had a normal distribution of collagen (Fig. 3A), whereas those treated with AFB1 demonstrated collagen deposition in a dose-dependent manner (p<0.05) (Fig. 3E). Table 3 summarizes the results of the histopathological analysis at each time point.

DISCUSSION
Many studies have shown that AFB1 exposure can lead to a reduction in weight gain in broiler chicks in a dose-and time-dependent manner (Valdivia et al., 2001; Tedesco et al., 2004; Zhao et al., 2010; Peng et al., 2014b; Fowler et al., 2015). Diaz et al (2008) proposed a biphasic nature (hormesis) of aflatoxins on the broiler’s weight gain, i.e. improvement at low doses (0.625 mg/kg and 1.25 mg/kg) and reduction at high doses (2.5 mg/kg and 5.0 mg/kg).
The results of the present study do not support this biphasic proposal of Diaz et al. (2008). Although at low doses (0.5 and 1.0 mg AFB1/kg of feed) there was no significant reduction, the body weights also did not increase compared with those in the control group until the end of the experiment (21 DPF). As exposure periods to aflatoxin B1 increased, body weight gain in the group fed 2.0 mg AFB1/kg of feed significantly decreased in a linear pattern beginning at 14 DPF.
Huff et al. (1986) reported that the relative liver weights decreased initially, but in our study, the relative liver weights significantly increased in the groups fed AFB1 at 1.0 and 2.0 mg/kg of feed beginning at 7 DPF. The relative liver weights also increased in a dose-dependent manner at 21 DPF (Fowler et al., 2015).
Similar to the results of previous studies, we found that the weights of the spleens were significantly increased during aflatoxicosis at 2 mg AFB1/kg of feed at 21 DPF (Huff et al., 1986; Peng et al., 2014a; Fowler et al., 2015).
At the cellular level, dietary AFB1 induced histopathological liver damage, including focal hepatocyte necrosis, hemorrhage, inflammatory cell infiltration, fibrosis, and nodular regeneration (Huff et al., 1986; Pandey et al., 2007; Tessari et al., 2010) in a dose- and time- dependent manner. The dose- dependent increase in the relative liver weights was similar to what was seen in the amount of liver fibrosis observed at 21 DPF.
At 21 DPF, the control group showed a slight amount of fatty degeneration, which is thought to be due to the rapid growth of the broiler. Although there was an increase in cellular fatty deposition, the gross liver color was difficult to distinguish (data not shown).
Serum biochemical and hematological alterations are also good tools for diagnosing chronic aflatoxicosis (Oğuz et al., 2000), because the detrimental effects on these values (Keceri et al., 1998) are apparent prior to the manifestation of clinical symptoms. The serum biochemical parameters and the effects caused by AFB1, however, has remained inconclusive.
Studies looking at the effects of AFB1 on serum chemistry have shown that serum cholesterol and total serum protein (TP) both decrease in birds fed a diet with 0.3 mg AFB1/kg of feed (Raju et al., 2000). Previous studies have also shown a decrease in the total serum protein and albumin levels at 1.0 mg AFB1/kg of feed, and a decrease in serum glucose, Ca++, and in organic P levels was reported in Ross-308 broiler chicks fed 2.0 mg AFB1/kg of feed at 21 days (Zhao et al., 2010).
We agree that aflatoxicosis negatively affects serum levels of total protein, albumin, and cholesterol (Huff et al., 1986; Zhao et al., 2010; Chen et al., 2014). In our experiments, the levels of TP, albumin, and cholesterol decreased significantly in the AFB1-fed group compared with the control group, but these changes varied with dose and duration. A significant decrease in serum cholesterol was seen at 14 DPF in the group fed 2.0 mg AFB1/kg of feed. A decrease in total protein was seen at 7 and 14 DPF in the group fed 2.0 mg AFB1/kg of feed. Albumin levels were lower at 7 DPF in the group fed 2.0 mg AFB1/kg and lower at 14 DPF in all of the AFB1-fed groups compared with the control group. Serum glucose levels in the group fed 0.5 mg AFB1/kg of feed were significantly higher compared with the other groups at 14 DPF, and levels at 21 DPF were significantly and dose-dependently reduced.
Calcium levels were significantly reduced in all of the AFB1-fed groups compared with the control at 14 DPF, but there was no change between the experimental groups at 21 DPF.
The inorganic P levels were significantly increased in the group fed 2.0 mg AFB1/kg of feed at 21 DPF, contrary to Zhao’s results (2010).
In the previous studies (Raju et al., 2000; Zhao et al., 2010), dietary aflatoxin showed no effect on serum AST or ALT levels. Yunus et al. (2011) reported that it was not possible to draw a dose-effect relationship for either AST or ALT levels. Our experiments, however, showed a significant dose-dependent increasement at 21 DPF.
Although the AST and ALT changes varied according to the AFB1 dose and exposure time (Tessari et al., 2010; Fowler et al., 2015; Hussain et al., 2016), we have shown that along with histological evaluation, serum values of AST, ALT, TP, glucose, and albumin may all serve as marker for chronic aflatoxicosis in poultry.
The data presented here indicate that both the dose of aflatoxin and the length of exposure influence the biochemical and histological response in broilers.








