INTRODUCTION
Avian leukosis virus (ALV) belongs to Group VI of the Retroviridae family and causes tumors and immunosuppression in avian species, resulting in significant economic losses. The ALV subgroups known to infect chickens are A-E, J, and K, and are classified depending on viral envelope proteins, which play important roles in interactions between host cells and the virus (Li et al., 2021). Host susceptibility and resistance to ALV are related to the interaction of viral glycoproteins with naturally occurring mutations of host receptors; hence, there are chicken lines that are naturally susceptible and resistant to ALV subgroups A-E, determined by three genetic loci: tva, tvb, and tvc (Barnard et al., 2006).
To infect host cells, ALV-A interacts with TVA, a receptor protein belonging to the low-density lipoprotein receptor (LDLR) family (Bates et al., 1993), and genetic variation of the TVA receptor in chickens determines susceptibility and resistance to ALV-A. A single nucleotide substitution in the LDLR-like domain of the tva gene (tvar1) changes the amino acid sequence from cysteine to tryptophan (C40W), thereby lowering the binding affinity of TVA for ALV-A. In another variant, insertion of four nucleotides into exon 1 of the tva gene (tvar2) results in a frameshift mutation that blocks TVA receptor expression, leading to infection resistance (Elleder et al., 2004). In addition, deletion of nucleotides in the intronic region of the chicken tva gene (tvar3, tvar4, tvar5, and tvar6) affects the branch point signal involved in splicing, resulting in incomplete mRNA splicing and decreased susceptibility to ALV-A (Reinisova et al., 2012; Chen et al., 2015). Further, recent findings demonstrate that ALV-K shares the TVA receptor with ALV-A (Prikryl et al., 2019), and knockout of Tva in chicken using the CRISPR/Cas9 system confers resistance to both ALV-A and -K (Koslova et al., 2021).
The TVB receptor protein belongs to the tumor necrosis factor receptor (TNFR) family and encodes a host receptor for ALV-B, -D, and -E (Adkins et al., 2000). Single nucleotide substitutions in the tvb gene regions encoding cysteine-rich domain (CDR) 1 (tvbr1) or CDR3 (tvbr3) induce premature stop codons that generate a truncated TVB receptor and decrease susceptibility of chickens to ALV-B, -D, and -E (Chen et al., 2017; Klucking et al., 2002). In addition, a G to C single nucleotide substitution (tvbr2) induces a cysteine to serine (C125S) amino acid alteration that lowers virus binding affinity (Reinisova et al., 2008), and insertions of two (tvbr4) or one (tvbr5) nucleotides in tvb exon 4 induces frameshift mutations, leading to expression of a truncated TVB receptor and reduced susceptibility to ALV-B, -D, and -E infection (Li et al., 2018). TVC is a member of the immunoglobulin superfamily, most similar to mammalian butyrophilins, and introduction of a premature stop codon in the gene encoding TVC confers resistance to ALV-C in chickens (Elleder et al., 2005).
ALV infection represents a substantial economic threat due to its promotion of tumor formation, growth retardation, immunosuppression, and decreased egg production. As ALV has a significant impact on the poultry industry, there have been many studies of Chinse chicken breeds to characterize ALV subgroup strains and the frequency of individuals with resistant alleles (Feng and Zhang, 2016; Su et al., 2018; Cui et al., 2020). Although molecular analysis of the genome of endogenous ALV has been performed in Korea (Kim et al., 2008), there have been no reports of virus resistance alleles in Korean Native chicken breeds. In this study, we analyzed the genome sequences of tva and tvb in the White Leghorn (WL), Korean Ogye (KO), and Korean Native chicken (KNC) breeds, to determine genotype frequencies and their resistance to ALV subgroups A, B, and K, with the aim of preserving superior traits by selective breeding in the future.
MATERIALS AND METHODS
The management and experimental use of chickens were approved by the Institute of Laboratory Animal Resources, Seoul National University, South Korea (SNU-190401-1-3). All experimental animals including White Leghorn (WL), Korean Ogye (KO), and Korean Native Chicken (KNC) were cared for according to a standard management program at the University Animal Farm, Seoul National University. The procedure for animal management and blood isolation adhered to the standard operating protocols of our laboratory.
Genomic DNA was extracted from blood sample from each chicken drawn from the wing vein. To identify genomic sequence of tva and tvb, genomic DNA was analyzed by PCR analysis using region-specific primers (TVA seq F: ACA CTG ACA GCG AGG CGT GC, TVA seq R: ACC TCT CCG CAC GAC GTT CT, TVB exon 3 seq F: TCT CCA CGT CTC GGC AGC AC, TVB exon 3 seq R: GGA GAG CCC GAG CAG AGC TG, TVB exon 4 seq F: GCT TTG GCA TGT GGG CAA GG, TVB exon 4 seq R: GCA GCA CGG TGA AGG TGA TG). For sequencing analysis, the PCR amplicons were cloned into the pGEM-T easy vector (Promega) and sequenced using an ABI Prism 3030XL DNA Analyzer (Thermo Fisher Scientific). The sequence was compared against assembled genomes using the Basic Local Alignment Search Tool (BLAST).
Total RNAs from WL, KO and KNC were isolated using Trizol Reagent (Thermo Fisher Scientific) and reverse-transcribed using the Superscript III First-Strand Synthesis System (Thermo Fisher Scientific). The cDNAs of WL, KO and KNCs were amplified with tva specific primer (TVA transcript F: CCG GCA TGG TGC GGT TGT TG, TVA transcript R: AGC CAG GTT CCA CGG TCA GC) by RT-PCR and tvb 5’ and 3’ specific primers (qRT Tvb 5’ F: TCT TCG CGG AGG TTC AGT, qRT Tvb 5’ R: TGG ATA CTC GGT GTA CTC GTC T, qRT Tvb 3’ F: TCC CAA AGT GGA AAC CC, qRT Tvb 3’ R: CTG CTC TGC CAG ATA AAG G) by RT-quantitative PCR (RT-qPCR). All reaction were performed under the same conditions, containing 100 ng cDNA, 20 Х EVA green (Biotium), 10 Х PCR buffer, 10 mM each of dNTP, 10 pM of each primer, and 0.5 U Taq polymerase (Bionics). RT-qPCR conditions were as follows: 95°C for 3 minutes followed by 40 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Melting-cure profiles were analyzed for all amplicons. Each test samples were run in triplicate. Relative quantification of the target gene expression we performed using the 2−ΔΔCt method using housekeeping gene GAPDH for control.
For chicken embryo fibroblast (CEF), fertilized eggs incubated for 6 days were isolated and dissected the limb, and organs from the embryo. The CEF were collected in a 1.5 mL microcentrifuge tube with 1 mL of 0.05% trypsin-EDTA and incubated at 37°C for 10 minutes. After adding the same volume of culture media, CEFs were centrifuged at 1,250 rpm for 5 minutes and the cells were maintained and sub-passaged in DMEM supplemented with 1 Х ABAM and 10% FBS. CEFs were incubated at 37°C with an atmosphere of 5% CO2 and 60%-70% relative humidity.
RCASBP-(A)-CN-EGFP, RCASBP-(B)-CN-EGFP and RCASBP-(K)-CN-EGFP was kindly provided by Dr. Yao and Dr. Nair (Pirbright Institute). Plasmid (5 μg) were mixed with Lipofectamine 2000 reagent (Thermo Fisher Scientific) in Opti-MEM (Thermo Fisher Scientific), and the mixture was applied to 1×106 DF-1 cells. The mixture was replaced culture medium 6 h after transfection. One day after transfection we could detect green fluorescence in DF-1 cells, which indicated virus production. Cells were subpassaged, and the medium was changed one day after subpassaging. One day later, the medium containing virus was harvested and frozen at −70°C until use. For viral infection, the medium containing virus was thawed at 37°C and added to individual CEF clones. Four days post-infection, WL, KO and KNC CEFs were observed using fluorescence microscopy (Tu-80; Nikon).
RESULTS
To analyze the genotype frequencies of the ALV receptors, tva and tvb, in WL, KO, and KNC, we sequenced four genetic loci associated with ALV susceptibility and resistance: tva exon 1, tva intron 1, tvb exon 3, and tvb exon 4 (Adkins et al., 2000; Elleder et al., 2004; Chen et al., 2015; Chen et al., 2017).
Analysis of tva loci revealed three genotypes, tvaS/S, tvaS/R2, and tvaS/R4, encoding TVA receptors for ALV-A and -K in WL. In exon 1, a susceptible allele (tvaS) and an allele with a 4 bp insertion (tvaR2) were observed, while in intron 1, we detected the susceptibility allele (tvaS) and a 5 bp deletion (tvaR4) in the variant region. Three resistance genotypes, tvaR2/R4, tvaR4/R5, and tvaR5/R5 (Elleder et al., 2004; Reinisova et al., 2012; Chen et al., 2015) were observed in KO, including the alleles tvaR2 and tvaR4, and a 10 bp deletion in the intronic region (tvaR5). For KNC, five tva genotypes (tvaS/S, tvaS/R4, tvaS/R5, tvaR4/R5, and tvaR5/R5) were observed (Figs. 1A and 1B). These results show that there are differences in tva genotypes and their frequencies among the three different Korean chicken breeds analyzed (Table 1).
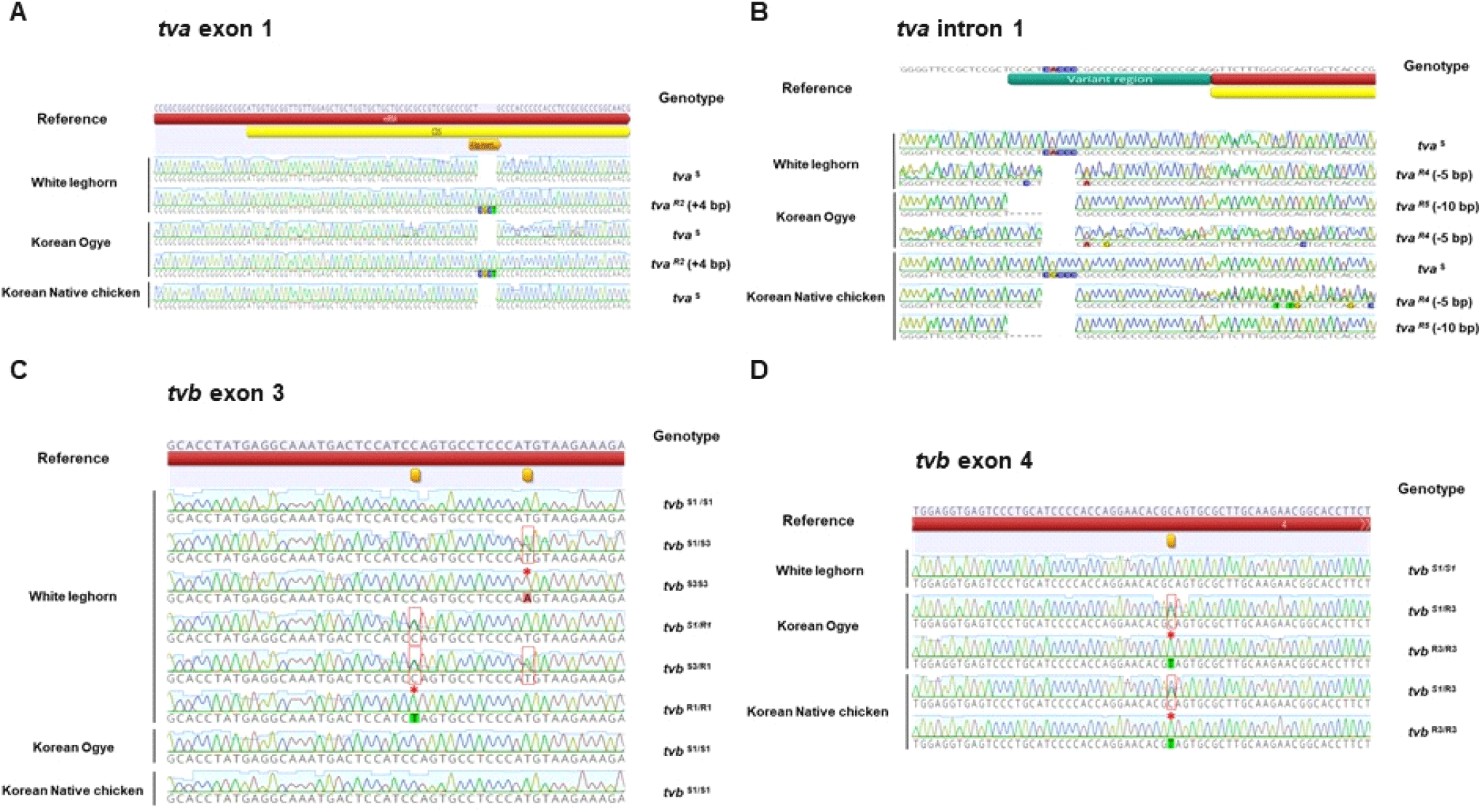
tvaS/S: susceptible homozygote for tva; tvaS/R2: heterozygote for CGCT insertion; tvaS/R4: heterozygote for ACCCC deletion; tvaS/R5: heterozygote for CGCTCACCCC deletion; tvaR2/R4: heterozygote for CGCT insertion and ACCCC deletion; tvaR4/R5: heterozygote for ACCCC deletion and CGCTCACCCC deletion; tvaR5/R5: homozygote for CGCTCACCCC deletion.
Next, we analyzed the TVB receptor, which is associated with ALV-B, -D, and -E, and found various susceptibility and resistance genotypes (tvbS1/S1, tvbS1/S3, tvbS3/S3, tvbS1/R1, tvbS3/R1, and tvbR1/R1) (Adkins et al., 2000) in WL. By contrast, in KO and KNC, we detected both heterozygosity (tvbS1/R3) and homozygosity (tvbR3/R3) for a resistance allele (tvbR3) encoding a premature stop codon in tvb exon 4 (Chen et al., 2017) (Figs. 1C and 1D). These results reveal that WL carries a variety of tvb genotypes, while a resistance allele dominates in KO and KNC through natural selection (Table 2).
tvbS1/S1 : susceptible homozygote for tvb; tvbS1/S3 : heterozygote for susceptible allele for tvb; tvbS3/S3 : susceptible homozygote for tvb, contains a SNP (184 T to A); tvbS1/R1, tvbS3/R1, tvbS1/R3 : heterozygote for susceptible and resistant allele; tvbR1/R1 : resistant homozygote for tvb, contains a SNP (172 C to T); tvbR3/R3 : resistant homozygote for tvb, contains premature stop codon in CRD2 domain (Q100*).
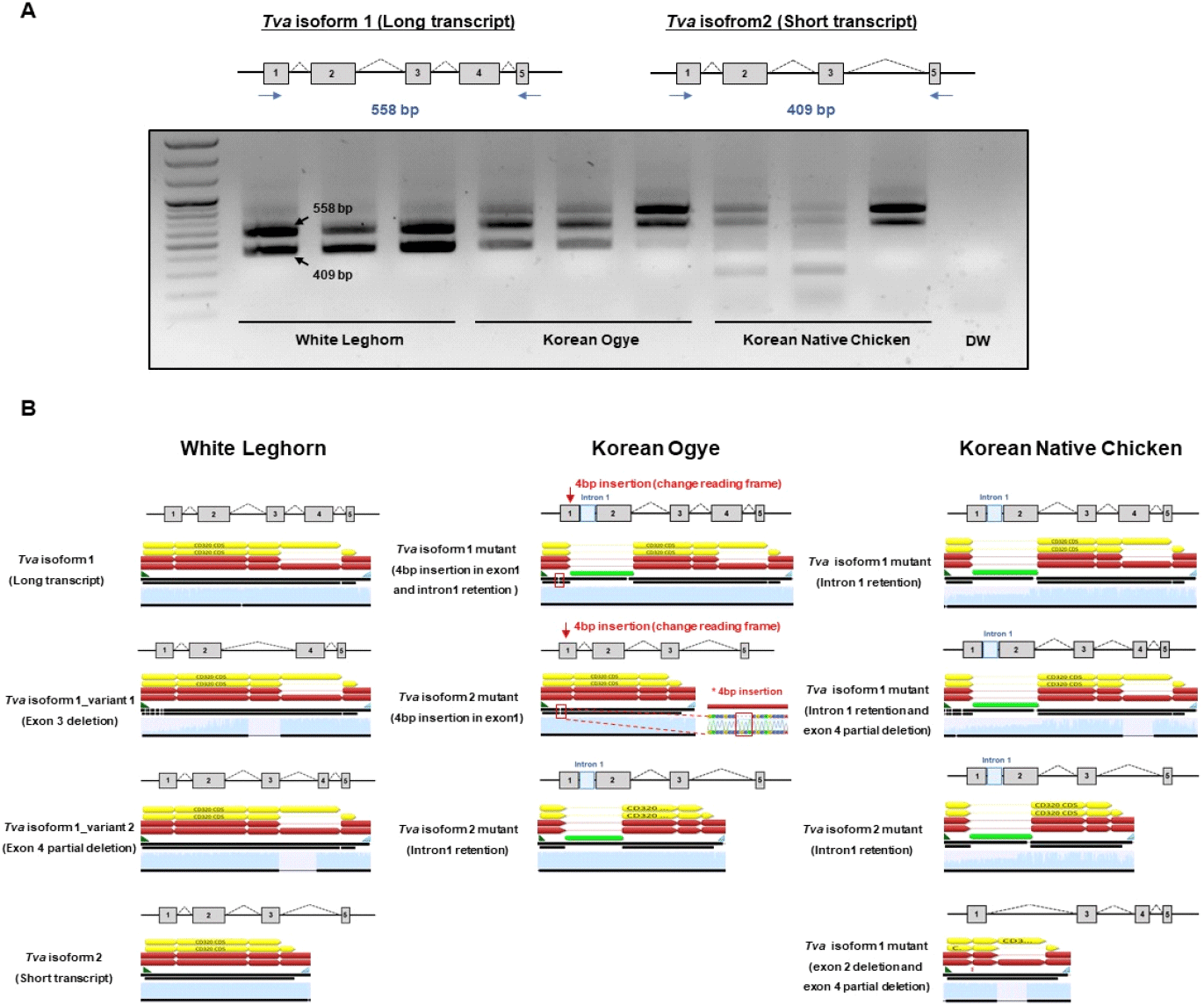
To determine the tva transcript splicing patterns in WL, KO, and KNC, we amplified the entire coding sequence of the tva gene by RT-PCR. For chickens with a tvaS/S genotype, a long tva transcript and a short transcript without a complete transmembrane domain were detected. Nucleotide deletions in the intronic variant region of the resistant alleles, tvaR4 and tvaR5, affect pre-mRNA splicing, resulting in retention of intron 1 in the mRNA transcript (Chen et al., 2015).
RT-PCR analysis of chicken embryo fibroblast (CEF) cDNA from WL, KO, and KNC showed that, unlike WL, multiple transcripts bands were observed in KO and KNC, due to incomplete splicing (Fig. 2A). Further sequencing analysis of the PCR products confirmed the presence of four types of transcript lacking intron 1 in WL. In KO, a 4 bp insertion in exon 1 containing intron 1 sequence, generated as a byproduct of incomplete splicing was observed. In KNC, intron 1 retention was observed in both the long and short transcripts, and a mutant form missing some exons was also detected (Fig. 2B).
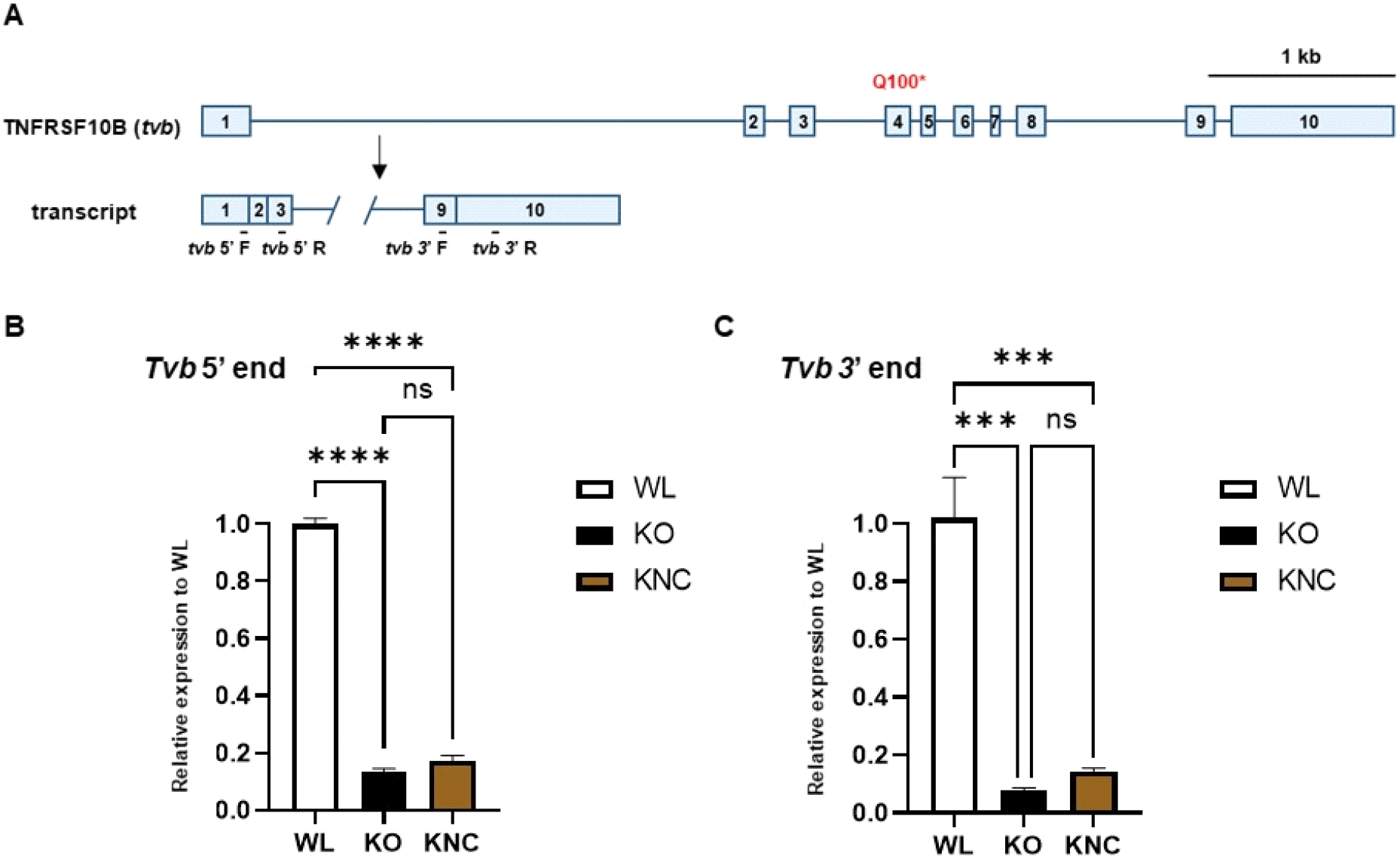
The tvbR3/R3 genotype, which is not present in WL, is predominant in KO and KNC, and generates a premature stop codon in exon 4, which induces nonsense-mediated RNA decay (Chen et al., 2017). To compare tvb transcript stability in a WL susceptible line, KO and KNC resistant lines, we designed primers to amplify the 5’ and 3’ termini of tvb (Fig. 3A). RT-qPCR analysis of both the tvb 5’ and 3’ ends of tvb in WL, KO, and KNC CEFs showed that transcript levels were significantly reduced in KO and KNC samples, relative to those from WL (Fig. 3B). These results show that the tvb transcript is degraded in KO and KNC, and that there are differences in the level of transcription of tvb in KO and KNC relative to WL.
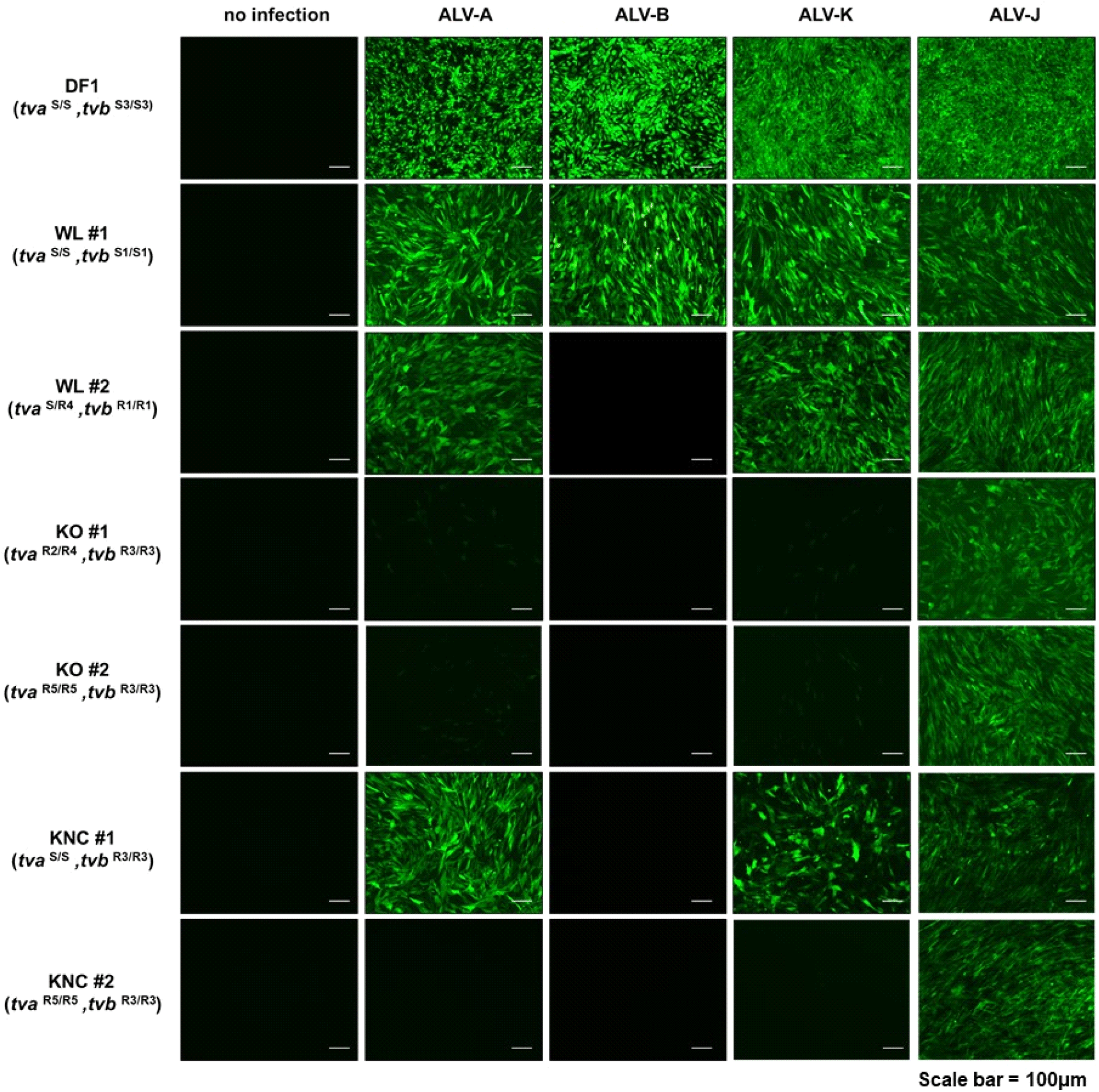
To verify the role of tva and tvb genotypes in resistance to ALV subgroups, we infected DF-1 chicken fibroblasts and each CEF clone with subgroup A, B, K, and J viruses. Expression of green fluorescent protein (GFP) was used as a marker to confirm viral replication, in DF-1 positive control cells and the WL #1 line (genotype: tvaS/S, tvbS1/S1), all ALV subgroups entered the cells and replicated. Our results revealed differences in resistance to ALV subgroups, even within the same breeds, according to their genotypes. ALV-B infection in WL line #2 (tvaS/R4, tvbR1/R1) did not lead to GFP expression, indicating that ALV-B could not replicate well in this CEF clone. In KO lines #1 and #2 (tvaR2/R4, tvbR3/R3 and tvaR5/R5, tvbR3/R3), in which both alleles of the tva and tvb genes confer ALV resistance, GFP expression was almost absent in cells infected with ALV-A, -B, and -K. Finally, ALV-B replication was blocked in KNC line #1 (tvaS/S, tvbR3/R3), whereas replication of ALV-A, -B, and -K was blocked in KNC line #2 (tvaR5/R5, tvbR3/R3). These results show that depending on the genotype, some KO and KNC breeds were naturally resistant to ALV-A, -B, and -K, whereas all breeds were susceptible to ALV-J (Fig. 4).
DISCUSSION
Multiple ALV subgroups, classified based on their glycoproteins, have significant impacts on the poultry industry. Although considerable efforts to eradicate and prevent ALV infection are ongoing, there is currently no vaccine that can adequately control ALV, and the continuous spread of ALV in Asian countries remains a problem (Feng and Zhang, 2016).
To inhibit viral replication in host cells, it is important to block the interaction between the viral glycoprotein and its host receptor. Indeed, there have been many reports that resistance to ALV subgroups can be naturally acquired by nucleotide mutations in host receptors. Single nucleotide polymorphisms in the genes encoding the TVA and TVB receptors affect viral binding affinity, while insertions or deletions of multiple nucleotides induce frameshift mutations, ultimately affecting viral resistance (Elleder et al., 2004; Reinisova et al., 2008; Reinisova et al., 2012; Chen et al., 2015; Li et al., 2018).
Our analysis revealed significant differences in genotypes and their frequencies among breeds. Genotypes comprising homozygous susceptibility and heterozygous susceptibility and resistance alleles for the TVA receptor were present in WL (tvaS/S, tvaS/R2, and tvaS/R4), while only resistance alleles were observed in KO (tvaR2/R4, tvaR4/R5, and tvaR5/R5), and susceptibility and resistance alleles were mixed in KNC (tvaS/S, tvaS/R4, tvaS/R5, tvaR4/R5, and tvaR5/R5). The difference in genotypes for the TVB receptor was more pronounced, with WL possessing various alleles (tvbS1/S1, tvbS1/S3, tvbS3/S3, tvbS1/R1, tvbS3/R1, and tvbR1/R1), whereas in KO and KNC, the resistant genotype (tvbR3/R3) was dominant, although some heterozygous genotype (tvbS1/R3) was detected.
A recent genome-wide analysis of KNC, Chinese indigenous breeds, and commercial breeds identified local adaptation and selection signatures related to innate immune responses in KO and KNC (Cho et al., 2022). In addition, a selection signature related to the innate immune response to Salmonella infection was identified in Korean indigenous goats using genome-wide analysis (Kim et al., 2019). Therefore, the natural occurrence of tva and tvb resistance alleles in KO and KNC can be considered selection signatures that have developed over long periods of time in the Korean Peninsula.
Recently reported data demonstrate that the avian TVA receptor protein belongs to the LDLR family, is an ortholog of mammalian CD320, and acts as a vitamin B12 transporter as well as a viral receptor (Krchlikova et al., 2021). Further, tva knockout chickens generated using CRISPR/Cas9 technology are not only resistant to ALV-A and -K, but also exhibit vitamin deficiency (Koslova et al., 2021). Growth retardation due to vitamin deficiency was not observed in KO and KNC individuals resistant to ALV subgroups, and further studies are needed to determine whether there are other genes that can compensate for lack of vitamin uptake by the TVA receptor.
The TVB receptor protein belongs to the TNFR family, which regulates dendritic cell and natural killer cell interactions apoptosis in response to DNA-damage stimulation, chronic inflammation and tumorigenesis induced in TNFR knockout mice (Finnberg et al., 2005; Finnberg et al., 2008; Iyori et al., 2011). TNFR is involved in several signaling pathways, but the function of the TNFR family member, tvb, in chicken remains unknown. Therefore, studies to determine whether other TNFR family genes are activated in KO and KNC in place of tvb are required. Both KO and KNC are resistant to ALV subgroups due to naturally occurring mutations in TVA and TVB proteins that have arisen during evolution; however, further studies are needed to validate the effects of these changes on the biological functions of TVA and TVB other than as viral receptors.
Lines naturally resistant to ALV-A, -B, and -K have been reported previously; however, no ALV-J resistant lines have been discovered in nature. Instead, an ALV-J resistant chicken, with viral binding disrupted by removal of the tryptophan 38 residue of the ALV-J receptor, was recently generated using the CRISPR/Cas9 system (Koslova et al., 2020). Therefore, construction of a multiple ALV resistant line, by selective breeding of the CRISPR/Cas9-generated ALV-J resistant line with naturally ALV-A, B, and K resistant lines, is expected to contribute greatly to the poultry industry.








