INTRODUCTION
Inflammation is a dynamic physiological response to infection caused by harmful pathogens (bacteria and viruses), damaged cells, injuries, chemical exposure, and prolonged stress (Wu et al., 2019). The inflammatory signaling cascade is triggered by pro-inflammatory cytokines such as interleukin (IL), tumor necrosis factor (TNF-α), and vascular endothelial growth factor (VEGF) (Wojdasiewicz et al., 2014). The prolonged or untreated inflammation may cause serious health conditions such as autoimmune disorders, chronic inflammation, neurodegenerative diseases, cancer, and other metabolic dysfunctions (Hajishengallis et al., 2021). The inflammatory response is mainly initiated after the recognition of pathogen-associated molecular patterns (PAMPs) by circulating immune cells and/or pattern recognition receptors (PRRs) in the innate and acquired immune system (Mendes et al., 2018). Next, the immune cells were orchestrated and activated the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling cascade (Peterson et al., 2014) through pro- inflammatory cytokines (e.g., IL-6, IL-8, interferon-γ) production and increasing the infiltration of heterophils and conscripting natural killer cells, macrophages, and T cells in the circulation. In addition, toll-like receptors (TLRs) are a major class of PRRs expressed on intestinal endothelial cells (IECs) and immune cells, which maintain both tolerance and inflammation against pathogens (Velová et al., 2018; Tarradas et al., 2020). Previously, Yu et al. (2018) reported that the mycoplasma-synthesized lipid-associated membrane protein stimulated IL-1β through TLR2 and myeloid differentiation factor 88 (MYD88) expression via the NF-κB inflammatory signaling pathway.
Inflammation in livestock, including poultry, has a major impact on the reliability of carcases and meat quality (Hafez et al., 2020). The multiple environmental factors, especially reused litter, intestinal pathogens, and changes in feed formulation, can trigger gut inflammation in poultry and impair nutrient absorption. Therefore, understanding inflammatory factors in poultry models may improve gut health and increase productivity (Pont et al., 2020). Therefore, there is a high demand for anti-inflammatory agents in the poultry industry. Synthetic anti-inflammatory drugs such as aspirin, statins, peroxisome proliferator-activated receptor (PPAR) agonists, and nonsteroidal compounds are commercially available because they are effective in treating inflammation. Alternatively, biologically derived metabolites have been developed as a potent anti-inflammatory agent that reduces the activity of secretory small molecules (anti-cytokine therapies) and regulates the inflammatory response of the innate immune system with improved safety (Dinarello, 2010).
Plant-derived natural compounds (phytochemicals) are one of the major sources for therapeutics that have the ability to control and treat various diseases, including immunomodulatory disorders, inflammation, oxidative stress, cancer, and respiratory disorders in humans and livestock (Newman and Cragg, 2020). Since a wide range of phytochemicals has been examined in many medicinal plants to enrich livestock health and function. Succulent plants have unique physiological characteristics that enable them to survive in extremely low moisture and high temperature conditions (Griffiths and Males, 2017; Niechayev et al., 2019). Succulents have important phytochemical potential such as antioxidant, neuro-protective, anti-inflammatory, biofuel, and biomass production (Juřica and Koupá, 2016; Males, 2017). Various edible succulents, such as aloe, yucca, and cactus, contain large amounts of nutrients (protein, fibre, vitamins, minerals, etc.) used as feed additives for development and immune function enhancement. Antioxidant and immunological properties have been reported in a small number of succulents, including aloe, saguaro fruit, dragon fruit, and prickly pear, and are commercially used in food due to their proven properties. In addition, many succulent plant species have been used throughout history to treat various local symptoms such as abdominal pain, burns, skin wounds, arthritis, and joint pain, but studies on their biological properties compared to ordinary medicinal plants are still insufficient. Recently, Aerts et al. (2017) examined the succulent plant Kalanchoe petitiana leaves to treat the metacarpal fracture in humans. The K. petitiana leaves were used to heat mildly and bandaged in the fractured area, which, according to ethnomedicine formulae, improves the fracture injury in hut keepers from rural mountain environments. Therefore, it is of interest to investigate succulents as a source of phytochemicals for the treatment of inflammation in chickens.
Dudleya brittonii (DB) is also one of the cactus flowering succulent plants in the family of Crassulaceae, which are widely grown in California, Mexico, and the United States. These succulent plants are found in dry and low water environments such as deserts, tropical regions, and coasts with high salinity (Griffiths and Males, 2017; Jang and Song, 2020). Sedeveria pink ruby (SP) is a cactus succulent plant that belongs to the Crassulaceae family. The Sedeveria genus is a hybrid of Echeveria and the Sedum genus. The plant leaves grow long and thick, storing high water content. We also found that still little is known about their biological mechanisms for humans and animals compared to other plant species/compounds. Interestingly, Kim et al. (2019) reported that an aqueous extract of DB protected against phorbol myristate acetate (PMA) -induced death in alveolar macrophages, which plays a role in preventing lung infection. DB extract inhibited the generation of reactive oxygen species and induced the expression of fatty acid oxidation and tricarboxylic acid cycle-related genes in DB-PMA-treated cells. However, the potential biological effects of DB and SP extracts on inflammation in chickens are still unclear. Therefore, here, we studied the anti-inflammatory activities of succulent plant extracts on DF-1 cells. DF-1 is a spontaneously immortalized chicken embryonic fibroblast (CEF) derived from the East Lansing Line (ELL-0) chicken embryo. DF-1 cells have good proliferation capacity than primary CEF cells and have TLR activity, so they are suitable as in vitro models for immune research in chickens (Lin et al., 2019). We investigated the effect of aqueous extracts of DB and SP on polyinosine:polycytidylic acid [poly(I:C)] -induced inflammation-associated gene and protein markers in DF-1 cells.
MATERIALS AND METHODS
DF-1 chicken (Gallus gallus) fibroblasts line (ATCC CRL-12203, ATCC, Manassas, VA, USA) was purchased. Cells were cultured with Dulbecco’s Modified Eagle Medium (DMEM, Biowest, Nuaille, France) supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic (Gibco, NE, USA) in a 37°C 5% CO2 atmosphere.
30 g of each DB and SP leaves were collected from the Department of Biotechnology, Hoseo University, Korea. Leaves were washed well with distilled water, mixed with 90 mL of sterile distilled water, and extracted at 110°C 39.23 kPa for 15 min in an autoclave (Daihan Scientific, Korea). The aqueous sample was centrifuged at 5000 RCF at 4°C for 10 min. The supernatant was collected, filtered by a 0.2 μm syringe filter (Sartorius, Goettingen, Germany), and stored at −20°C for further use (stock: 70 mg/mL) (Kim et al., 2019). Serial diluted solutions were from 10−1 to 10−9 (7 mg/mL to 7 pg/mL).
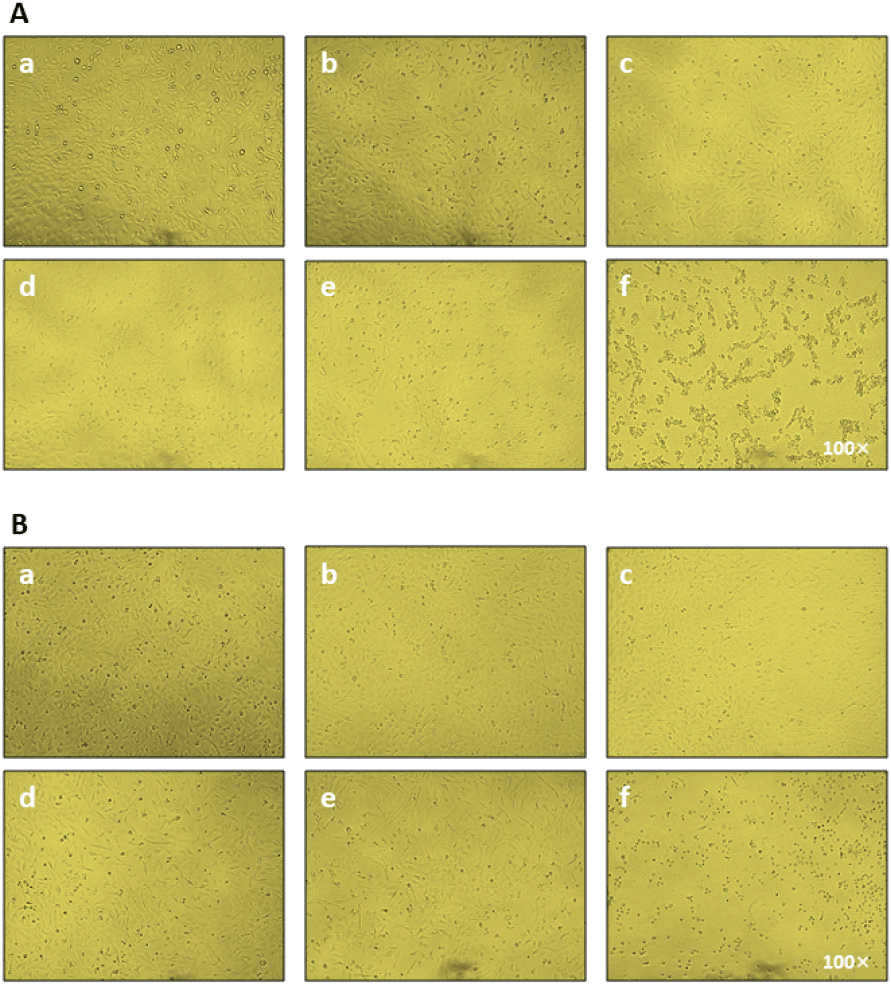
Cell viability was determined by using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assay as previously described (Laksmitawati et al., 2017). In brief, DF-1 cells were seeded on a 96-well plate with a cell density of 1 × 104 cells/mL. Poly(I:C), a synthetic analogue of double-stranded RNA (dsRNA), was purchased (InvivoGen, San Diego, CA, USA) and pre-treated at 60°C for 10 min to increase the reactivity for 24 hr. The poly (I:C) was dissolved in phosphate-buffered saline (PBS, pH 7.4) at a concentration of 100 μg/mL, and aliquots were prepared and stored at −20°C. The different concentrations of each plant extract (10−1 to 10−9 dilution) and poly(I:C) (1—100 μg/mL) were prepared freshly. The cells were treated with different concentrations of extracts and the drug for 12 hr and 24 hr, respectively. After incubation time, 30 μL MTS reagent was added to each well and the plate was incubated for 3 hr to convert MTS into the aqueous soluble formazan product by the dehydrogenase enzyme secreted by the metabolically active cells present in the samples. The absorbance was measured at 490 nm using a microplate reader (Thermo Fisher Scientific, Waltham, MA, USA), because the absorbance value is directly proportional to the percentage of viable cells in each well. The morphologies and viability of DF-1 cells treated with plant extracts (DB and SP) were examined for 48 hr and images were captured using a light microscope (Olympus IX73).
DF-1 cells were seeded at a density of 5 × 105 cells/mL in a 6-well plate. After confluence, the cells were treated for 18 hr with various amounts of plant extracts, followed by 6 hr with 5 μg/mL poly(I:C) to induce inflammation in the cultured cells. The treatments in this study were divided into six groups based on the extracts, concentrations, and non- treated control group; Group 1: Control (untreated), Group 2: 5 μg/mL poly(I:C) alone, Group 3: 7 μg/mL DB extract with 5 μg/mL poly(I:C), Group 4: 7 pg/mL DB extract with 5 μg/mL poly(I:C), Group 5: 7 μg/ml SP extract with 5 μg/mL poly(I:C), Group 6: 7 pg/mL SP extract with 5 μg/mL poly(I:C). The samples were collected after 24 hr treatment and stored at −20°C for further analysis.
Total RNA was extracted from DF-1 cells using PureLinkTM RNA Mini kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA quantity was measured by spectrophotometry at 260/280 nm, and 0.5—1 μg of each RNA sample was reverse-transcribed to cDNA using a cDNA synthesis kit (ReverTra Ace-α-Kit, Toyobo, Osaka, Japan) as per the manufacturer’s guidelines. Quantitative real-time PCR (qPCR) was performed to examine the gene expression profile in test and control samples of DF-cells. A 10 ng of cDNA was used to conduct the qPCR reaction with iTaqTM Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA). The target gene-specific primers used in the study are listed in Table 1. The qPCR was performed in a qPCR system (C1000M Thermal Cycler, Bio-Rad, CA, USA), and relative quantification of target gene expression was calculated using the 2−ΔΔCt method, where ΔΔCt = (Ct of the target gene-Ct of the GAPDH) treatment − (Ct of the target gene — Ct of GAPDH) control (Livak and Schmittgen, 2001).
The numerical results are presented as mean ± standard error (SE) (Microsoft Excel 2007, Microsoft, Redmond, WA, USA) of three independent experiments. Statistical analysis was carried out by SPSS16.0 (IBM, San Diego, CA, USA). One-way ANOVA analysis of variance followed by post-hoc Duncan’s test was used to measure the P-value. Data are considered significant when the P-value is less than 0.05.
RESULTS
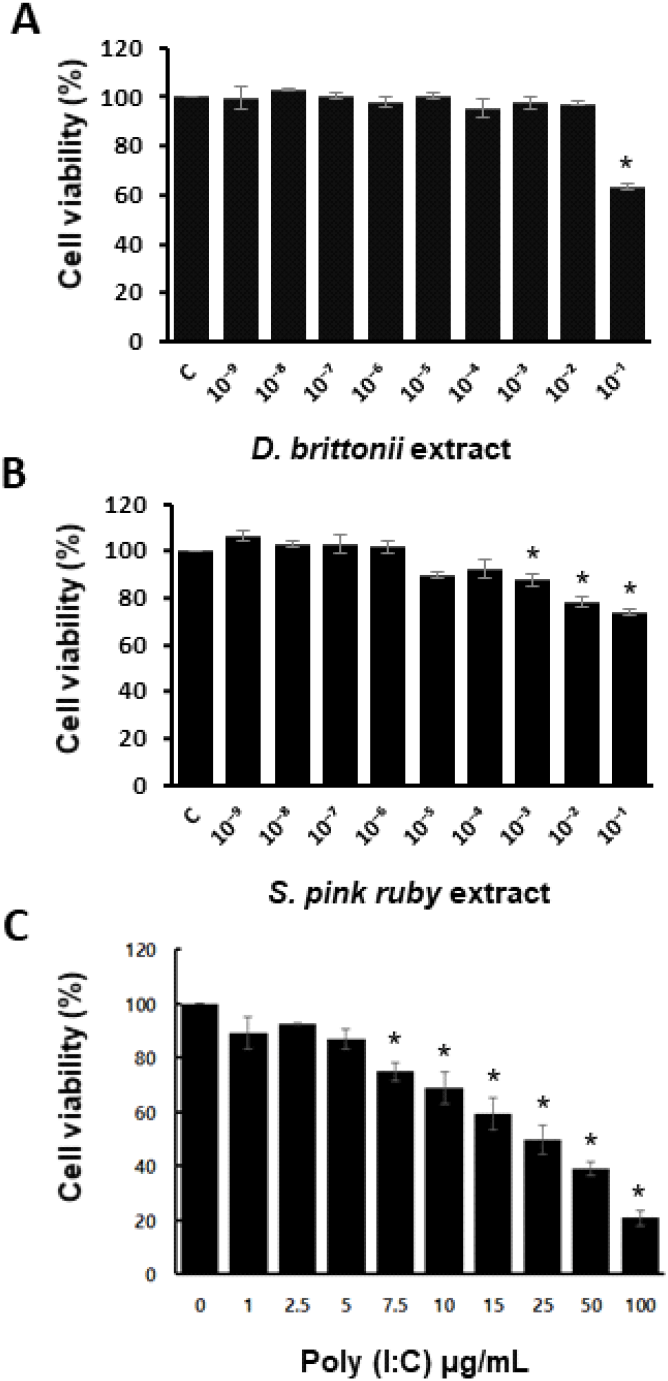
To determine whether aqueous extracts of DB and SP affect cell viability in poly(I:C) treated DF-1 cells, an MTS assay was performed. Plant extract dilutions (10−9—10−1 dilutions) were prepared and tested for dose-dependent cell viability. Up to a 10−4 dilution, both plant extracts showed no cytotoxicity (Figs. 1A and 1B). SP extract was cytotoxic at 70 μg/mL (10−3 dilution), whereas DB extract was cytotoxic at 7 mg/mL (10−1 dilution) (P<0.05). We treated cells with different doses of poly(I:C) from 1 to 100 μg/mL for 24 hr to determine the optimum concentration (Fig. 2C). The MTS experiment revealed that poly(I:C) treatment significantly reduced cell viability at concentrations of 7.5 μg/mL and higher (Fig. 2C, P<0.05). As a result, we used a poly(I:C) concentration of 5 μg/mL throughout the investigation. Both DB and SP extracts reduced the number of viable DF-1 cells at a high dose of 7 mg/mL (10−1 dilution) after 24 hr of exposure (Figs. 1A and 1B). A close examination of plant extract-treated cells revealed that greater doses altered cell shape and viability more than lower dilutions (Figs. 2A and 2B). We selected the optimal doses of the plant extract to assess anti-inflammatory activity in DF-1 cells (7 μg/mL and 70 pg/mL) throughout the study.
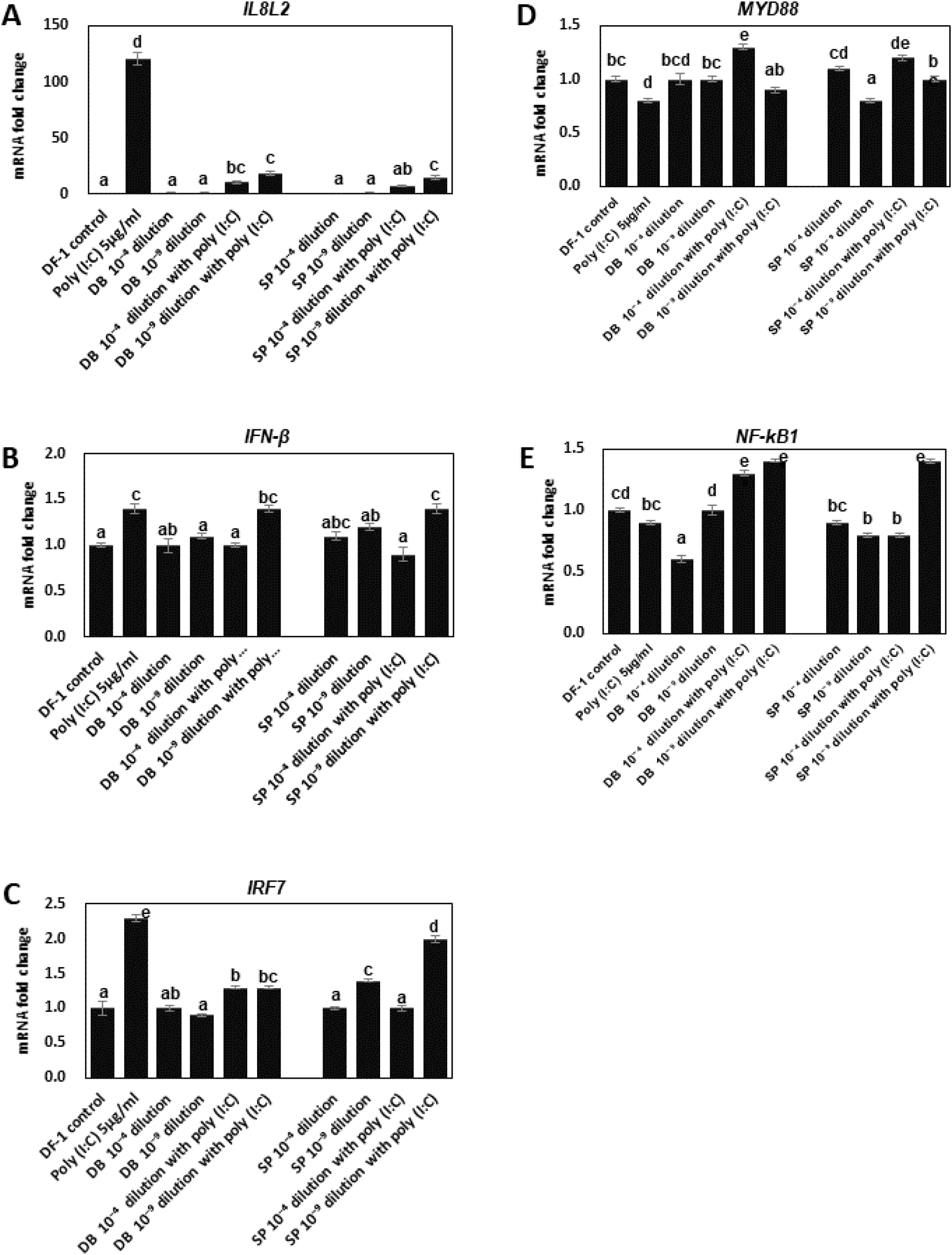
In DF-1 cells cultured in the presence of poly(I:C) or combinations with DB, qPCR was used to examine the changes in gene expression of inflammatory mediators such as interleukin 8-like 2 (IL8L2) and interferon beta (IFN-β), proinflammatory cytokines, and interferon regulatory factor 7 (IRF7), a transcription factor for inflammation. In DF-1 cells, poly(I:C) treatment increased the expression of IL8L2 (Fig. 3A), IFN-β (Fig. 3B), and IRF7 (P<0.05, Fig. 3C), demonstrating that DF-1 cells may be used to assess the anti-inflammatory activity of plant extracts. DB and SP treatment suppressed poly(I:C)-induced inflammatory gene expressions in DF-1 cells. Expressions of IL8L2,IFN-β, and IRF7 were decreased by both DB and SP treatment compared to the poly(I:C) alone group (Fig. 3). DB aqueous extract treatments inhibited poly(I:C)-induced IL8L2 and IFN-β expressions most effectively at a dose of 7 μg/mL (P<0.05, Figs. 3A and 3B). Poly(I:C)-induced IRF7 expression was also inhibited by DB extracts at the doses of 7 μg/mL and 70 pg/mL (Fig. 3C). In addition, we examined whether DB and SP extracts alter the expressions of MYD88 and NF-κB1 genes in combination of poly(I:C). Expressions of MYD88 were repressed by poly(I:C), but were not altered by both DB and SP extracts (Fig. 3D). It is of note that in combination with poly(I:C), either high doses of DB or SP extracts de-repressed the expression of MYD88 (Fig. 3D). Poly(I:C) reduced NF-κB1 expression, but not significantly (P>0.05, Fig. 4E). A high dose of DB extracts repressed NF-κB1 expression but not SP extract, but this repression was markedly reversed by a combination of poly(I:C) with either DB or SP extracts (Fig. 3E). NF-κB1 expression was repressed by a high dose of DB extract but not by a low dose, but this repression was significantly reversed by combining poly(I:C) with either a high or a low dose of DB extract (Fig. 3E). It is worth noting that when combined with poly(I:C), either a high dose of DB or SP extracts suppressed NF-κB1 expression (Fig. 3E)
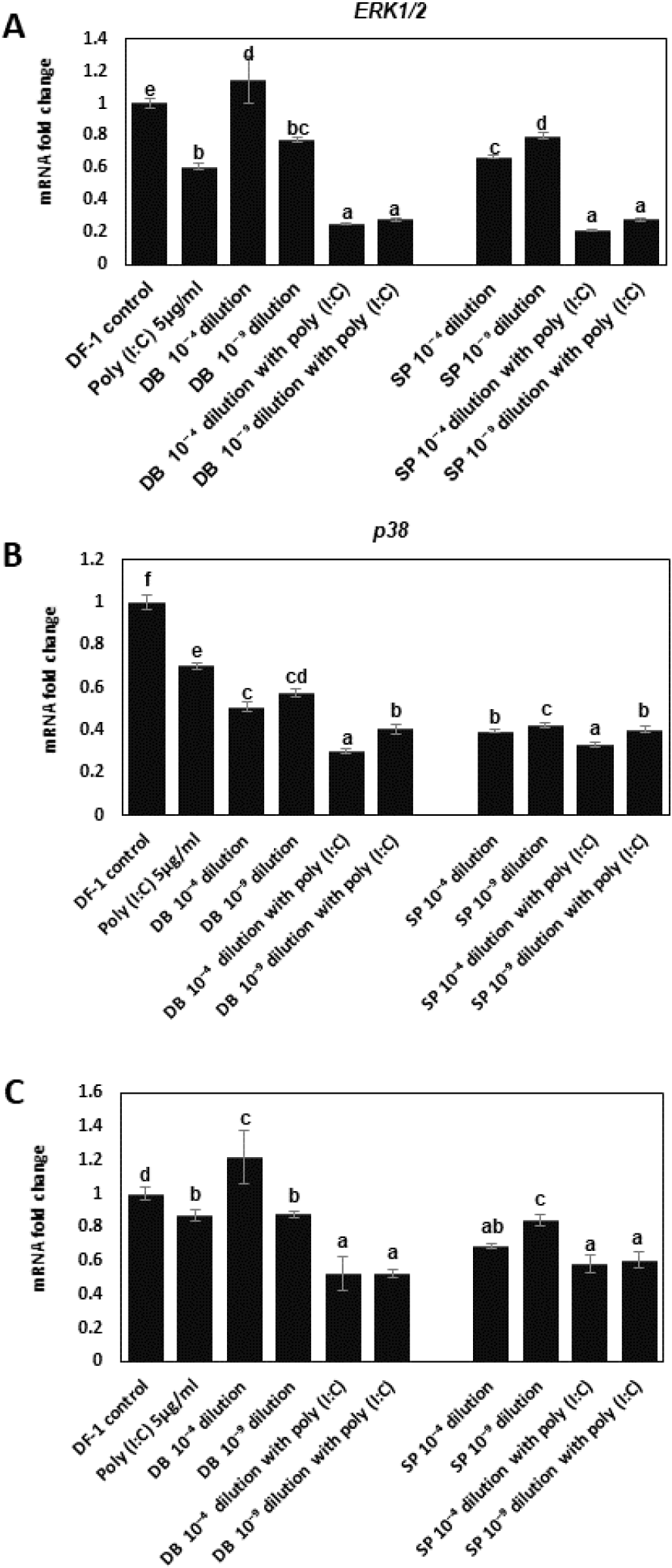
qPCR was used to investigate the effect of DB and SP extract on the transcription of mitogen-activated protein kinases (MAPKs) genes in DF-1 cells. TLR3 activation significantly decreased extracellular signal-regulated kinase 1/2 (ERK1/2), p38 MAPK, and c-Jun N-terminal kinase (JNK) expressions in DF-1 cells (P<0.05, Figs. 4A, 4B, and 4C). qPCR was used to investigate the effects of SP and DB extracts on the transcription of MAPK genes in DF-1 cells, specifically ERK1/2, p38 MAPK, and JNK. TLR3 activation significantly reduced transcription of ERK1/2, p38 MAPK, and JNK in DF-1 cells (P<0.05, Fig. 4). At a low dose, DB and SP treatments inhibited the expression of ERK1/2 and JNK genes (P<0.05, Figs. 4A and 4C). A high dose of DB had no effect on the expression of ERK1/2 or JNK genes (P>0.05), but a low dose of SP suppressed ERK1/2 expression (P<0.05, Figs. 4A and 4C). Regardless of dose, treatment with poly I:C) and DP or SP extracts further suppressed the expression of ERK1/2 and JNK genes (P<0.05, Figs. 4A and 4C). Treatment with either DP or SP extracts suppressed p38 MAPK gene expression and, when combined with poly(I:C), further suppressed p38 MAPK expression (P<0.05, Fig. 4B). In summary, these succulent extracts possess the capacity to modulate the transcription of inflammatory and MAPK signaling genes in chicken cells, suggesting these extracts could be promising candidates for potent natural anti-inflammatory agents for poultry caused by viral infections.
DISCUSSION
We evaluated the biological activities of two succulent plant extracts, i.e., DB and SP extracts, in DF-1 chicken cells, as well as the transcriptional responses of proinflammatory and MPAK genes in these cells, in this study. MTS assay was used to investigate the cytotoxicity of succulent plant extracts on DF-1 cells at various doses (70 pg/mL to 7 mg/mL). Cell viability by DB and SP extracts were dramatically affected when concentrations were increased from 70 pg/mL to 7 mg/mL, compared to non-treated cells. In addition, our findings show that when the concentration of plant extracts increases, the percentage of viable cells decreases (Figs. 1 and 2).
In this study, we investigated the effect of aqueous extracts of DB and SP against poly(I:C) induced inflammation in DF-1 cells. DB and SP extracts inhibited the poly(I:C)- induced expression of proinflammatory genes (IL8L2, IFN-β, and IRF7). A high and low dose of DB extracts, on the other hand, increased the expression of MYD88 and NF-κB1 in poly(I:C). In addition, a low dose of SP extracts increased NF-κB1 expression with poly(I:C). Inflammation is induced by innate immune receptors, including PRRs, TLRs, and Nod-like receptors (NLRs), which detect pathogens and immune signaling molecules to maintain cellular functions and tissue homeostasis (Chovatiya and Medzhitov, 2015; Kogut et al., 2018). However, chronic inflammation and hyperinflammation are toxic to maintaining homeostasis. The inhibitory effects of plant extracts and plant-derived compounds on the expression of pro-inflammatory genes have been reported and continue to prove their potential as natural anti-inflammatory agents. Schink et al. (2018) showed that the ethanolic plant extracts contain anti-inflammatory compounds that reduce the NF-κB expression and p65 translocation in THP-1 monocytes and HeLa-TLR4 cells. Chimonanthus nitens Oliv. leaf extract dose-dependently suppresses the lipopolysaccharide (LPS) -induced pro-inflammatory cytokine (TNF-α, IL-6, and IL-1β) production in RAW 264.7 cells (Sun et al., 2017). Li et al. (2017) suggested that Radix isatidis isolated polysaccharides develop the immune system and induce the production of IgG antibodies, which are used as antiviral adjuvants. And R. isatidis isolated polysaccharides reduce the pro-inflammatory cytokines (TNF-α and IL6) in the LPS-stimulated mouse pneumonia model (Laura et al., 2021). In this study, DB and SP extracts exhibited inhibitory effects on pro-inflammatory gene expression characteristic of plant-derived anti-inflammatory agents. The immunomodulatory effect of plant extract has also been demonstrated in chickens. Plant extracts supplementation significantly reduces mRNA levels of IL-6 and TNF-α in hens (Xie et al., 2019). Liu et al. (2019) reported that rutin plant derivatives could attenuate LPS-induced inflammation in chicken muscle cells, and suggested supplementation of rutin with plant extracts as a promising approach to reduce the risk of infection in broilers. In addition, the formulated plant-based feed for chicken may induce an immune-modulatory activity and reduce the risk of developing chronic inflammation and viral infections (Li et al., 2017).
In this study, poly(I:C) induced IRF7 expression was downregulated by DB and SP extracts. The IRF transcription factor family is critical for the regulation of many aspects of immune responses, including immune cell development and differentiation, as well as antimicrobial and viral responses, particularly those mediated by PRRs (Honda and Taniguchi, 2006). Each of the nine members of the family has a well-conserved DNA-binding domain that recognizes IFN-stimulated response elements (ISREs) in target gene promoters. When cytosolic PRRs are activated, type I IFN genes (the genes that encode IFN-α and IFN-β) are typically triggered. IRF7 is the principal regulator of these genes’ induction, while IRF3 also plays a part in this process. Both IRF3 and IRF7 are phosphorylated by TBK1 (TANK-binding kinase 1) and converted into active forms (Honda and Taniguchi, 2006). Unlike in mammals, IRF3 is absent in the chicken genome; however, IRF7 plays a critical role in inducing type I interferon in response to viral infections (Cormican et al., 2009; Kin and Zhou, 2018). Gain- and loss-of-function studies on the IRF7 gene revealed that IRF7 plays a critical role in the replication of Newcastle disease (Wang et al., 2014) and avian influenza virus (Kim and Zhou, 2015) by altering IFN signaling pathways. Only a few studies reported the transcriptional regulation of the IRF7 gene. It has not been studied whether DB and SP extracts directly or indirectly regulate the IRF7 transcriptions in DF-1 cells, as these plant extracts are mixtures of catechins and polyphenols (Kim et al., 2019).
The MAPK cascades are intracellular signal transduction pathways that respond to a variety of extracellular stimuli and regulate a wide range of fundamental cellular processes such as growth, proliferation, differentiation, motility, stress response, survival, and apoptosis (Plotnikov et al., 2011). The MAPK signaling cascade also plays a key role in the initiation of the inflammatory reaction. The MAPK family is made up of four prototype members: ERK1/2, p38 MAPK, JNK or stress-activated protein kinase (SAPK), and ERK5, which are activated by specific MAPKKs: MAP2K1/2 for ERK1/2, MKK3/6 for the p38 MAPK family, MKK4/7 (JNKK1/2) for the JNKs, and MAP2K5 for ERK5. The first MAPK pathway discovered was the ERK1/2 cascade (Seger and Krebs, 1995), which is now used as a model for these kinase cascades. This cascade is required for the signaling of a wide variety of extracellular agents, such as growth factors, which act via various receptors. The p38 MAPKs are activated by environmental factors such as oxidative and osmotic stress, UV and gamma radiation, cytokines, and inflammatory signals. The p38 MAPKs, which are activated by MAPKKs, MKK-3, and MKK6, may control proinflammatory responses to stimulation, leading to an increase in the expression of RANTES (regulated upon activation, normal T cell expressed and presumably secreted), IL-8, and TNF (Cargnello and Roux, 2011; Canovas and Nebreda, 2021). JNKs are made up of three genes (JNK1, JNK2, and JNK3) that are activated by different external stimuli, as well as the initial signaling pathway (Dhanasekaran and Reddy, 2008). JNKs activate transcription factors such as NF-B1/2, ETS transcription factor ELK4 (ELK-4), c-Fos, activator protein-1 (AP-1), activating transcription factor 2 (ATF2), p53, JunD, and c-Jun, all of which play important roles in controlling the immune response to viral infection and increasing the production of pro-inflammatory cytokines such as TNF, IFN, IL-1, IL-2, and IL-6. Previously, Kaji (2010) suggested that the Lactobacillus strain in food could positively induce the production of IL-10 and IL-12 anti-inflammatory cytokines through MAPK activation in mouse macrophage cells. The L. plantarum strains showed rapid activation of ERK, p38 and JNK than the L. casei. Similarly, Lactobacillus lipoteichoic acid (LTA) probiotics stimulate the regulatory cytokines (i.e., IL-10 and IL-12) via p44/42 signaling, resulting in an anti-inflammatory response (Zadeh et al., 2012). In contrast, the MAPK signaling cascade, such as p38, ERK 1/2, and JNK, is inhibited by the expression of inflammatory cytokines, which are associated with the innate immune system, and through the activation of IFN-β downstream signaling (Lee et al., 2020; Eo et al., 2021). Resveratrol is a natural compound with strong anti-inflammatory activity in a dose-effect relationship. It suppresses the TLR4 and MyD88 expression in activated macrophage cells. Also, the natural compound inhibits NF-kB, MAPK, IRF3 transcription factors, and ROS enzymes (Tong et al., 2020).
In this study, DB and SP extracts possess the capacity to modulate the expressions of MAPK genes, i.e., ERK1/2, p38 MAPK, and JNK, which were altered by TLR stimulation in the presence of either these plant extracts with or without poly(I:C) (Fig. 4). It should be noticed that in the presence of both DB and SP extracts with or without poly(I:C), transcription of MAPK genes was suppressed (Fig. 4). These findings suggested that SP and DB have the potential to treat inflammation in chickens by modulating the expression of MAPK genes at the transcription level. Further investigation warrants identifying the single compounds that target transcriptional alterations of the genes that were examined in this study.
This study showed that aqueous extract of succulent plants (S. pink ruby and D. brittonii) attenuated the poly(I:C)-induced inflammation signaling in DF-1 cells. In DF-1 cells, the DB and SP extracts down-regulated the inflammation-related marker genes as well as MAPK genes with or without TLR3 stimulation, suggesting that DB and SP are potential sources for modulating inflammation by pro-inflammatory and MAPK signaling pathways. Therefore, further study with DB and SP extracts warrants identifying the single compounds which may be responsible for transcription of pro-inflammatory and MAPKs signaling pathway genes, whose transcriptions are altered by these plant extracts, and to test whether these plant extracts can regulate the modifications of signaling proteins as well as protein levels.
In this study, we aimed to evaluate the anti-inflammatory activity of succulents [Dudleya brittonii (DB) and Sedeveria pink ruby (SP)] against poly(I:C) induced DF-1 chicken fibroblasts in vitro. DB and SP extracts exhibited anti-inflammatory activity at concentrations below 70 μg/mL (10−3 dilution), which were not toxic to DF-1 cells. Both DB and SP extracts significantly reduced pro-inflammatory gene expressions that were induced by poly(I:C), and DB and SP extracts also strongly reduced mRNA expressions of MAPK genes in the DF-1 cells treated compared with the non-treated group, suggesting that DB and SP extracts are valuable plant resources for inflammation control in chicken.








